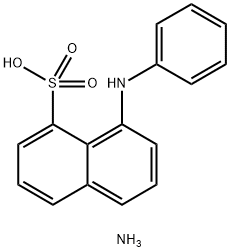
8-Anilinonaphthalene-1-sulfonic acid ammonium salt:Preparation process and application
General Description
8-Anilinonaphthalene-1-sulfonic acid ammonium salt, is an environmentally sensitive and a fluorescent anionic probe.[1]8-Anilinonaphthalene-1-sulfonic acid ammonium salt has optical properties, i.e. light absorption and luminescence characteristics, which make it useful as probes of polarity in many proteins and enzymes. 8-Anilinonaphthalene-1-sulfonic acid ammonium salt dissolved in water exhibits the fluorescence characteristics of a high-polarity medium, while the solutions of the dye in various alcohols display the corresponding characteristics of intermediate or lowpolarity media.[2]

Figure 1 8-Anilinonaphthalene-1-sulfonic acid ammonium salt tablet powder
Preparation
preparation method of high-purity 8-anilino-1-naphthalene sulfonic acid comprises the following steps: The preparation method has a simple process flow, is low in cost and low in environmental pollution, and can be amplified experimentally; and the industrial production with annual output of more than 1,000kg can be achieved. High-purity 8-anilino-1-naphthalene sulfonic acid prepared by the method and the salt product have high purity, contain less impurities, and can meet the application requirement of the biotechnology field.The preparation of high-purity 8-anilino-1-naphthalenesulfonic acid includes the following steps:
Step 1:Refining and processing domestic industrial grade citric acid, aniline and sulfuric acid respectively to obtain refined citric acid, aniline and sulfuric acid. The refined aniline and the refined pericyclic acid undergo a condensation reaction in the presence of refined sulfuric acid. The mass ratio of the aniline to the pericyclic acid is 1: 2.06 to 2.08. The mass ratio of the sulfuric acid is 1:1.1-3.0; the reaction temperature is controlled between 15-60℃, the continuous reaction time is 24-36h, and a condensation reaction product is obtained, and the condensation reaction product is distilled under reduced pressure to obtain 8-anilino-.The crude product of 1-naphthalenesulfonic acid is dark green or emerald green.
Step 2:Prepare 10% by volume of sodium hydroxide solution and measure 100mL and pour into a container. With stirring, slowly add 85g of 8-anilino-1-naphthalenesulfonic acid crude product to the sodium hydroxide solution. After the crude product of 8-anilino-1-naphthalenesulfonic acid is completely dissolved, 17-20g of neutral alumina and 8-10 g of activated carbon are added to the reaction system, and after stirring for two hours, it is filtered through ordinary filter paper and 0.2 nm filter membrane , Filter three times and collect the filtrate.
Step 3:Pour the filtrate collected in step 2 into a clean container; prepare a 50% by volume sulfuric acid solution and pour into a dropping funnel, adjust the valve to slowly drop the 50% by volume sulfuric acid solution into the Stirring in the filtrate; the mass of the sulfuric acid dropped is 1/3 of the mass of the 8-anilino-1-naphthalenesulfonic acid described in Step 2. The dropping speed of sulfuric acid must be slow, the speed is 0.5-2ml/s ; Then filter through ordinary filter paper and 0.2nm filter membrane to collect the first filter residue.
Step 4:Prepare 20% by volume sulfuric acid solution; according to 3:1 volume ratio (20% by volume sulfuric acid solution:8-anilino-1-naphthalenesulfonic acid solution=3:1,8-anilino-1-naphthalene The volume of the sulfonic acid solution is the volume of the filtrate obtained in step 2) The first filter residue is washed with the 20% volume sulfuric acid solution, and then filtered through ordinary filter paper and a 0.2 nm filter membrane, repeated three times, and the second filter residue is collected.
Step 5:Prepare 10% by volume sulfuric acid solution, and then wash the second filter residue with the 10% by volume sulfuric acid solution according to the same volume ratio in step 4, and then filter through ordinary filter paper and 0.2nm filter membrane, repeat three times, collect A third filter residue is obtained.Wash the third filter residue with pure water, repeatedly suction filter until the pH value of the filtrate is about 6-7, collect the fourth filter residue, and then place the fourth filter residue in a vacuum drying oven and dry at 45-60°C for 24-30h, to obtain purified 8-anilino-1-naphthalenesulfonic acid.[3]
Application
The 8-Anilinonaphthalene-1-sulfonic acid ammonium salt was used as a fluorescent probe to determine the surface hydrophobicity of zein hydrolysates.[4]
Reference
[1]Yang C, Liu Y, Li Q, et al. Interaction of Three Non-steroidal Anti-inflammatory Drugs with Egg Phosphatidylcholine Liposomes[J]. Acta Physico-Chimica Sinica, 2007, 23(5): 635-639.
[2]Liu M, Wang F, Pu C, et al. Nanoencapsulation of lutein within lipid-based delivery systems: Characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier[J]. Food Chemistry, 2021, 358: 129840.
[3]Yuan Z,Zhang J. A process for preparing 8-phenylamino-1-naphthalene sulfonic acid and its salts[P].2012,CN102757369.
[4]Liu M, Wang F, Pu C, et al. Nanoencapsulation of lutein within lipid-based delivery systems: Characterization and comparison of zein peptide stabilized nano-emulsion, solid lipid nanoparticle, and nano-structured lipid carrier[J]. Food Chemistry, 2021, 358: 129840.